More than 3.7 million patients globally use UCB medicines. In the U.S., we reached more than 400,000 patients in 2021. As a leading global biopharmaceutical company, we embrace the possibility of creating solutions for patients to manage the burdens of diseases such as those affecting the skin.
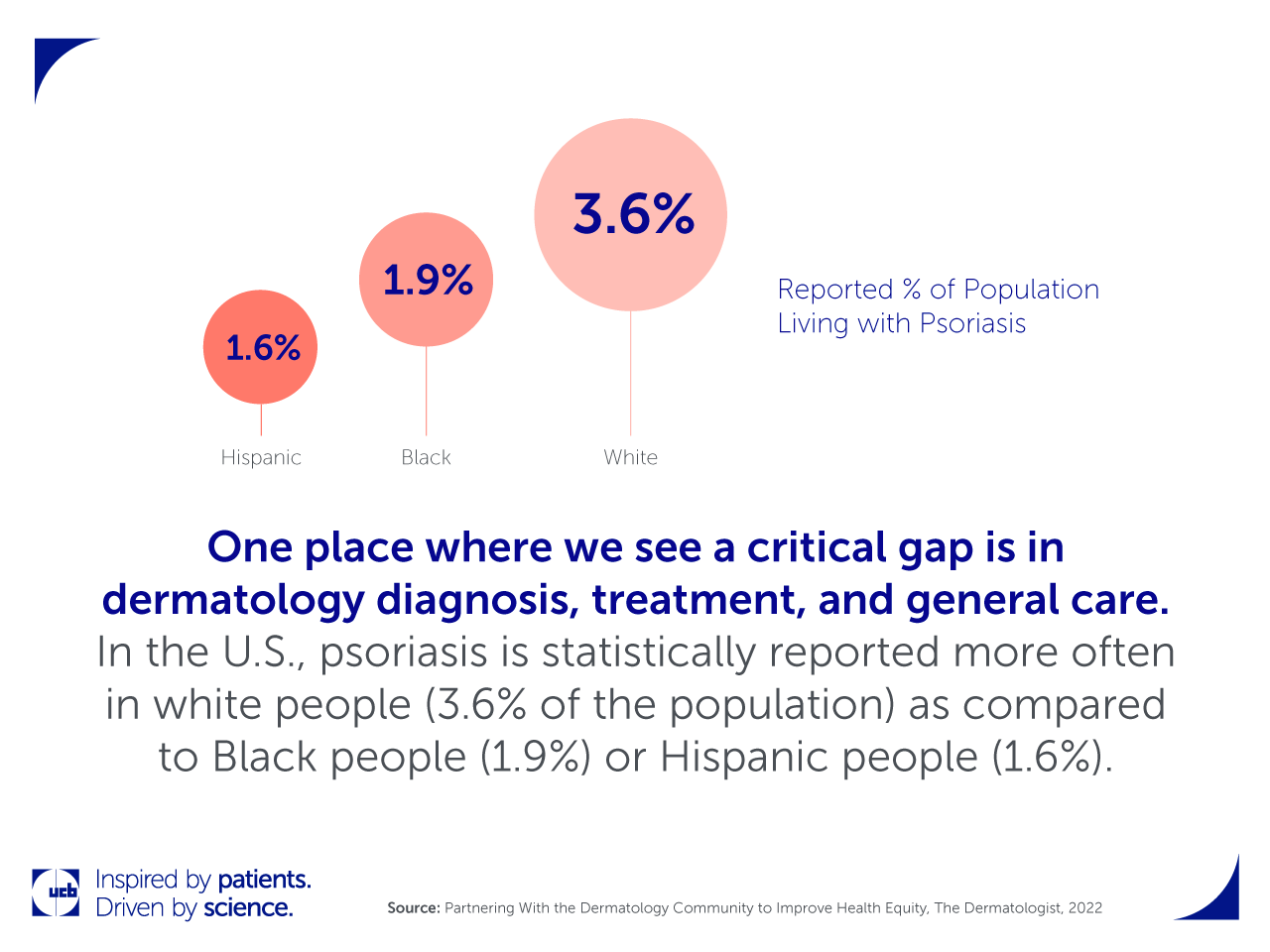
One place where we see a critical gap is in dermatology diagnosis, treatment, and general care. In the U.S., psoriasis is statistically reported more often in white people (3.6% of the population) as compared to Black people (1.9%) or Hispanic people (1.6%).1 However, the prevalence of psoriasis may actually be higher in people of color because of how the disease presents itself and is clinically treated in people with darker skin tones. Not only does this impact diagnosis, but it also affects the types of treatments offered, access to those treatments, and most importantly, the patient’s quality of life.
At UCB, we understand we have an important role to play in helping to close gaps impacting historically excluded and disenfranchised populations. We believe that health equity is achieved when every person can attain their full health potential, and no one is disadvantaged from achieving this potential because of social position or other socially determined circumstances.
Our goal is to help achieve equity across the care continuum from research to the delivery of innovative medicines. Diversity and inclusion in dermatological clinical trials is critical. People with skin of color are drastically underrepresented in clinical trials.2 The lack of diversity in clinical trials means that medications developed for conditions like psoriasis may not work as effectively for people with skin of color. Ultimately, the health equity gap continues to widen.
We are committed to working toward a clinical trial infrastructure that addresses health disparities and closes the gap in clinical trial diversity to better reflect the intended treatment population. Implementing decentralized clinical trials (DCTs) is changing how we approach the design of our trials by focusing on reducing overall in-person patient visits and increasing remote-friendly visits. Across our portfolio, UCB has approximately 14% of active studies in the DCT model, with more than half of those study visits being fully remote. We’re hopeful approaches like DCTs can help ensure access to studies for a broader range of participants by removing barriers such as distance, transportation, and time required.
Another barrier is information about clinical trials and a clear path for enrolling. Past clinical trial enrollment processes have often been too burdensome and not easily understandable. In addition, many people simply do not know of clinical trials or how to enroll. To solve for this, our clinical operations teams are trained to include diversity considerations in every clinical trial. We also work with Site Engagement Managers in local communities to help raise awareness and encourage participation in clinical trials. This includes working with physicians and staff at clinical trial sites. We are leveraging ways to find and connect with diverse patient populations by using digital and data-driven approaches to address health inequities. Approaches like decentralized clinical trials, use of real-world data, and personalized tools to enhance the patient experience are helping us innovate our ways of working.
The practice of medicine hinges on a provider’s ability to connect with patients and to understand the factors that influence their lives and their health outcomes. UCB believes people living with dermatological conditions achieve the best possible health outcomes when healthcare providers practice cultural awareness and humility. Not only that, but the overall industry is better served when all medical staff and industry leaders like UCB practice these same traits.
Originally introduced in 1998 by pediatricians and public health practitioners Melanie Tervalon and Jann Murray-Garcia, cultural humility is a dynamic and lifelong process focusing on self-reflection and personal critique as well as acknowledging one’s own biases. Culture is expansive and is not limited to racial or ethnic identity – it includes language, location, religion, sexual orientation, gender identity/expression, and more. Practicing cultural humility is a willingness to learn from patients as valued experts on their lived experiences while recognizing and respecting a patient’s intersecting identities.
UCB recognizes the imperative to connect personally by developing one-on-one relationships with patients. We regularly engage with patient communities, listening and learning from advocacy and professional organizations to help us shape our clinical development programs and patient support materials. We take every opportunity, whether at a medical congress or a local community event, to meet patients to better understand their needs. By considering the needs of diverse populations through deep patient listening and practicing cultural humility, UCB can continue to improve representation in clinical studies and insights.
At UCB, we are serious about addressing health equity through population health efforts and actions. UCB is a true partner within the dermatology community – committed to going beyond the medicine to improve the lives of traditionally underserved patients. But we know we can’t go it alone. Partnership and collaboration are key. It is up to all of us to identify more ways to improve outcomes for those groups who have been historically marginalized. We can do this together!
References:
- WebMD. How Psoriasis Affects People of Color. Available at https://www.webmd.com/skin-problems-and-treatments/psoriasis/psoriasis-people-of-color. Accessed November 2022.
- Cavazzoni P, Anagnostiadis E, Lolic M. 2020 drug trials snapshots summary report. U.S. Food & Drug Administration. Available at https://www.fda.gov/media/145718/download. Accessed November 2022.
Choose Country
- Global Site – English
- Australia – English
- België – Engels
- Belgique – Anglais
- Brasil – Português
- България – Български
- Canada – English
- Canada – Français
- 中国 – 中文
- Česká Republika – Angličtina
- Danmark – Engelsk
- Deutschland – Deutsch
- France – Français
- España – Español
- Ελλάδα – Ελληνικά
- India – English
- Ireland – English
- Italia – Inglese
- 日本 – 日本語
- Казахстан – ағылшын тілі
- 한국 – 한국어
- Luxembourg – Anglais
- Luxemburg – Engels
- Magyarország – Angol
- México & Latinoamérica – Español
- Nederland – Engels
- New Zeeland – English
- Norge – Engelsk
- Österreich – Deutsch
- Polska – Polski
- Portugal – Inglês
- România – Engleză
- Россия – Русский
- Slovensko – Anglický
- Suomi – Englanti
- Sverige – Engelska
- Schweiz – Deutsch
- Suisse – Français
- Türkiye – Türkçe
- Україна – Англійська
- United Kingdom – English
- U.S.A. – English


