This Press Release is Intended for Media and Investor Stakeholders Only
- Diverse and patient-focused data set comprises 17 abstracts including one oral presentation
- Features new data analyses for UCB’s generalized myasthenia gravis (gMG) treatments, including post hoc and open-label extension results from the pivotal Phase 3 MycarinG study and additional Interim Analyses of RAISE-XT for the approved treatments RYSTIGGO®▼ (rozanolixizumab-noli) and ZILBRYSQ®▼ (zilucoplan)
- Data on BRIVIACT® (brivaracetam), FINTEPLA®▼ (fenfluramine), and STACCATO® alprazolam showcase commitment to patients and momentum of UCB’s epilepsy and rare syndromes portfolio
ATLANTA, April 12, 2024: 07:00 (ET) -- UCB (Euronext Brussels: UCB), a global biopharmaceutical company, today announced the latest research from its expansive neurology portfolio and pipeline to be presented at the 76th American Academy of Neurology (AAN) Annual Meeting, April 13-18, 2024, in Denver, Colorado, USA.
A total of 17 abstracts, including an oral presentation, will feature data from studies of four approved and one investigational medicine and technologies for generalized myasthenia gravis (gMG), epilepsies including Dravet syndrome and focal (partial) epileptic seizures.
Key UCB scientific and patient-focused data to be presented at AAN include:
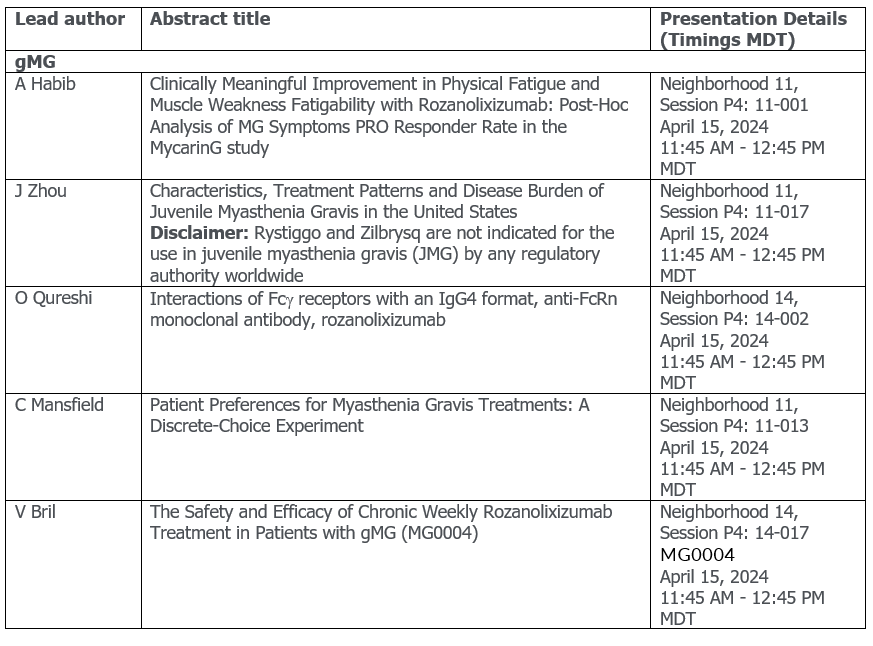
gMG
- Post hoc and open-label extension analyses from the pivotal Phase 3 MycarinG study for rozanolixizumab, including impact of treatment on physical fatigue and muscle fatigability in adult patients with gMG
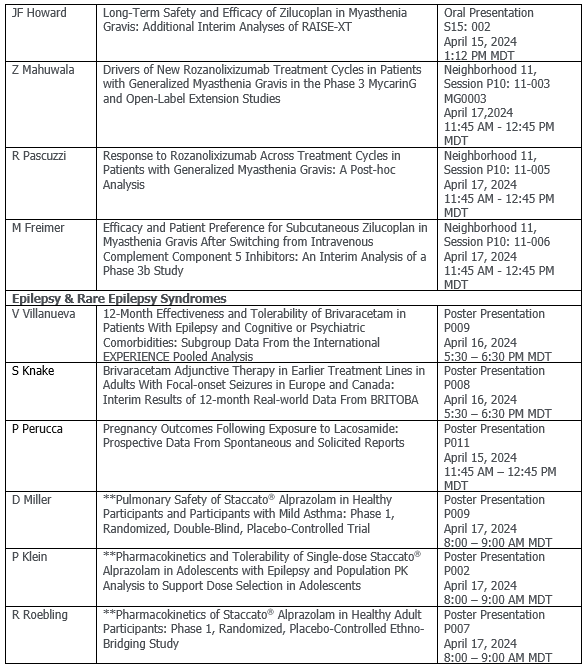
- An oral presentation on long-term safety and efficacy of zilucoplan in gMG in an interim analysis of RAISE-XT
- An interim analysis of a Phase 3b study on efficacy, and patient preference for subcutaneous zilucoplan in Myasthenia Gravis after switching from intravenous complement component 5 inhibitors
- Results from a discrete choice study looking at patient preferences in gMG treatment attributes
Epilepsy and rare epilepsy syndromes
- Focal-onset seizures (FOS) - interim results of 12-month real-world study (BRITOBA) evaluating adjunctive brivaracetam in earlier treatment lines in adults
- Epilepsy - subgroup data from the international EXPERIENCE analysis assessing brivaracetam in epilepsy patients with cognitive or learning disability or psychiatric comorbidities
- Prolonged seizures - three Phase 1 studies evaluating the safety, tolerability, and pharmacokinetics of single-use inhaled alprazolam (an investigational treatment for potential termination of prolonged epileptic seizures) in different populations
- Dravet syndrome - a retrospective analysis using US claims data of healthcare utilization and persistence in patients with Dravet syndrome
- Epilepsy disease management - expert consensus recommendations from the Seizure Termination Project*, highlighting best practice for rapid and early seizure termination and timing for intervention
“The new analyses from the Phase 3 MycarinG and Phase 3 RAISE XT open-label extension (OLE) studies being presented at this year’s AAN meeting reinforce the potential of our recently approved gMG treatments, RYSTIGGO®▼ and ZILBRYSQ®▼, to offer targeted treatments for gMG patients, tailored to their individual needs and preferences,” commented Donatello Crocetta, Head of Global Rare Disease & Rare Medical, UCB. “These data further underscore UCB’s innovative approach to evolving science into meaningful treatment solutions that help improve long term patient outcomes of people living with this rare neuromuscular disease, and more widely as part of the integrated neurology portfolio’s commitment to patient-focused solutions.”
“The data being presented at this year's AAN meeting showcase how we are transforming experiences and outcomes for people living with epilepsy and rare epilepsy syndromes and highlight our work in redefining the future of epilepsy care,” said Mike Davis, Global Head of Epilepsy & Rare Syndromes, UCB. “Everything we do is centered around people and families living with epilepsies, helping them achieve their ideal and maximize their life opportunities.”
*The Seizure Termination Project was funded by UCB Pharma.
**STACCATO® alprazolam is an investigational product. The safety and efficacy has not been established and it is not approved by the Food and Drug Administration, or by any health authority worldwide.
For further information, contact UCB:
Global Rare Disease Communications
Jim Baxter
T+ 32.2.473.78.85.01
jim.baxter@ucb.com
Global Communications
Nick Francis
T +44 7769 307745
email nick.francis@ucb.com
Rare Disease Communications
Daphne Teo
T +1 (770) 880-7655
email daphne.teo@ucb.com
Epilepsy and Rare Syndromes Communications
Becky Malone
T +1 (919) 605-9600
email becky.malone@ucb.com
Corporate Communications, Media Relations
Laurent Schots
T+32.2.559.92.64
Laurent.schots@ucb.com
Investor Relations
Antje Witte
T +32.2.559.94.14
antje.witte@ucb.com
Important Safety Information about RYSTIGGO® (rozanolixizumab-noli) in the US1
RYSTIGGO (rozanolixizumab-noli) is a neonatal Fc receptor blocker indicated for the treatment of generalized myasthenia gravis (gMG) in adult patients who are anti-acetylcholine receptor (AChR) or antimuscle-specific tyrosine kinase (MuSK) antibody positive.6
WARNINGS AND PRECAUTIONS
Infections: RYSTIGGO may increase the risk of infection. Delay RYSTIGGO administration in patients with an active infection until the infection is resolved. During treatment with RYSTIGGO, monitor for clinical signs and symptoms of infection. If serious infection occurs, administer appropriate treatment and consider withholding RYSTIGGO until the infection has resolved.
Immunization
Immunization with vaccines during RYSTIGGO treatment has not been studied. The safety of immunization with live or live-attenuated vaccines and the response to immunization with any vaccine are unknown. Because RYSTIGGO causes a reduction in IgG levels, vaccination with live-attenuated or live vaccines is not recommended during treatment with RYSTIGGO. Evaluate the need to administer age-appropriate vaccines according to immunization guidelines before initiation of a new treatment cycle with RYSTIGGO.
Aseptic Meningitis: Serious adverse reactions of aseptic meningitis (also called drug-induced aseptic meningitis) have been reported in patients treated with RYSTIGGO. If symptoms consistent with aseptic meningitis develop, diagnostic workup and treatment should be initiated according to the standard of care.
Hypersensitivity Reactions: Hypersensitivity reactions, including angioedema and rash, were observed in patients treated with RYSTIGGO. Management of hypersensitivity reactions depends on the type and severity of the reaction. Monitor patients during treatment with RYSTIGGO and for 15 minutes after for clinical signs and symptoms of hypersensitivity reactions. If a reaction occurs, institute appropriate measures if needed.
ADVERSE REACTIONS
In a placebo-controlled study, the most common adverse reactions (reported in at least 10% of RYSTIGGO-treated patients) were headache, infections, diarrhea, pyrexia, hypersensitivity reactions, and nausea. Serious infections were reported in 4% of patients treated with RYSTIGGO. Three fatal cases of pneumonia were identified, caused by COVID-19 infection in two patients and an unknown pathogen in one patient. Six cases of infections led to discontinuation of RYSTIGGO.
The full Prescribing Information is available at https://www.ucb-usa.com/RYSTIGGO-prescribing-information.pdf
Important Safety Information about ZILBRYSQ® (zilucoplan) in the US2
IMPORTANT SAFETY INFORMATION INCLUDING BOXED WARNING
ZILBRYSQ is a complement inhibitor indicated for the treatment of generalized myasthenia gravis (gMG) in adult patients who are anti-acetylcholine receptor (AChR) antibody positive.7
INDICATION
ZILBRYSQ (zilucoplan) is indicated for the treatment of generalized myasthenia gravis (gMG) in adult patients who are anti-acetylcholine receptor (AChR) antibody positive.
IMPORTANT SAFETY INFORMATION INCLUDING BOXED WARNING
WARNING: SERIOUS MENINGOCOCCAL INFECTIONS
Life-threatening and fatal meningococcal infections have occurred in patients treated with complement inhibitors; ZILBRYSQ is a complement inhibitor. Meningococcal infection may become rapidly life-threatening or fatal if not recognized and treated early.
- Complete or update meningococcal vaccination (for serogroups A, C, W, and Y, and serogroup B) at least 2 weeks prior to administering the first dose of ZILBRYSQ, unless the risk of delaying therapy outweighs the risk of developing a meningococcal infection. Comply with the most current Advisory Committee on Immunization Practices (ACIP) recommendations for meningococcal vaccinations in patients receiving a complement inhibitor.
- Persons receiving ZILBRYSQ are at increased risk for invasive disease caused by N. meningitidis, even if they develop antibodies following vaccination. Monitor patients for signs of meningococcal infections and evaluate immediately if infection is suspected.
Because of the risk of serious meningococcal infections, ZILBRYSQ is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called ZILBRYSQ REMS.
CONTRAINDICATIONS
ZILBRYSQ is contraindicated in patients with unresolved Neisseria meningitidis infection.
WARNINGS AND PRECAUTIONS
Serious Meningococcal Infections
Life-threatening and fatal meningococcal infections have occurred in both vaccinated and unvaccinated patients treated with complement inhibitors; ZILBRYSQ is a complement inhibitor. The use of ZILBRYSQ increases a patient’s susceptibility to serious and life-threatening meningococcal infections (septicemia and/or meningitis) caused by any serogroup, including non-groupable strains.
Complete or update meningococcal vaccination (for both serogroups A, C, W, and Y [MenACWY] and serogroup B [MenB]) at least 2 weeks prior to administering the first dose of ZILBRYSQ, according to current ACIP recommendations for meningococcal vaccinations in patients receiving a complement inhibitor.
If urgent ZILBRYSQ therapy is indicated in a patient who is not up to date with both MenACWY and MenB vaccines according to ACIP recommendations, administer meningococcal vaccine(s) as soon as possible and provide the patient with antibacterial drug prophylaxis.
Closely monitor patients for early signs and symptoms of meningococcal infection and evaluate patients immediately if infection is suspected. Withhold administration of ZILBRYSQ in patients who are undergoing treatment for meningococcal infection until the infection is resolved.
ZILBRYSQ REMS
Due to the risk of meningococcal infections, ZILBRYSQ is available only through a restricted program under a REMS called ZILBRYSQ REMS.
Under the ZILBRYSQ REMS, prescribers must enroll in the program. Prescribers must counsel patients about the risk of meningococcal infection, provide the patients with the REMS educational materials, and ensure patients are vaccinated with meningococcal vaccines. Additional information on the REMS requirements is available at www.ZILBRYSQREMS.com or 1-877-414-8353.
Other Infections
ZILBRYSQ blocks terminal complement activation; therefore, patients may have increased susceptibility to infections, especially with encapsulated bacteria, such as infections caused by Neisseria meningitidis but also Streptococcus pneumoniae, Haemophilus influenzae, and to a lesser extent, Neisseria gonorrhoeae. Administer vaccinations for the prevention of Streptococcus pneumoniae and Haemophilus influenzae type b (Hib) infections according to ACIP guidelines. Persons receiving ZILBRYSQ are at increased risk for infections due to these bacteria, even after vaccination.
Pancreatitis And Other Pancreatic Conditions
Pancreatitis and pancreatic cysts have been reported in patients treated with ZILBRYSQ. Patients should be informed of this risk before starting ZILBRYSQ. Obtain lipase and amylase levels at baseline before starting treatment with ZILBRYSQ. Discontinue ZILBRYSQ in patients with suspected pancreatitis and initiate appropriate management until pancreatitis is ruled out or has resolved.
ADVERSE REACTIONS
In a placebo-controlled study, the most common adverse reactions (reported in at least 10% of gMG patients treated with ZILBRYSQ) were injection site reactions, upper respiratory tract infections, and diarrhea.
Please see the full Prescribing Information for additional Important Safety Information.
Important Safety Information about FINTEPLA® (fenfluramine) in the US3
INDICATIONS AND USAGE
FINTEPLA is indicated for the treatment of seizures associated with Dravet syndrome (DS) and Lennox-Gastaut syndrome (LGS) in patients 2 years of age and older.
IMPORTANT SAFETY INFORMATION
BOXED WARNING: VALVULAR HEART DISEASE and PULMONARY ARTERIAL HYPERTENSION
- There is an association between serotonergic drugs with 5-HT2B receptor agonist activity, including fenfluramine (the active ingredient in FINTEPLA), and valvular heart disease and pulmonary arterial hypertension.
- Echocardiogram assessments are required before, during, and after treatment with FINTEPLA.
- FINTEPLA is available only through a restricted program called the FINTEPLA REMS.
CONTRAINDICATIONS
FINTEPLA is contraindicated in patients with hypersensitivity to fenfluramine or any of the excipients in FINTEPLA and with concomitant use, or within 14 days of the administration, of monoamine oxidase inhibitors because of an increased risk of serotonin syndrome.
WARNINGS AND PRECAUTIONS
Valvular Heart Disease and Pulmonary Arterial Hypertension (see Boxed Warning): Because of the association between serotonergic drugs with 5 HT2B receptor agonist activity, including fenfluramine (the active ingredient in FINTEPLA), and valvular heart disease (VHD) and pulmonary arterial hypertension (PAH), cardiac monitoring via echocardiogram is required prior to starting treatment, during treatment, and after treatment with FINTEPLA concludes. Cardiac monitoring via echocardiogram can aid in early detection of these conditions. In clinical trials for DS and LGS of up to 3 years in duration, no patient receiving FINTEPLA developed VHD or PAH.
Monitoring: Prior to starting treatment, patients must undergo an echocardiogram to evaluate for VHD and PAH. Echocardiograms should be repeated every 6 months, and once at 3-6 months post treatment with FINTEPLA.
The prescriber must consider the benefits versus the risks of initiating or continuing treatment with FINTEPLA if any of the following signs are observed via echocardiogram: valvular abnormality or new abnormality; VHD indicated by mild or greater aortic regurgitation or moderate or greater mitral regurgitation, with additional characteristics of VHD (eg, valve thickening or restrictive valve motion); PAH indicated by elevated right heart/pulmonary artery pressure (PASP >35 mmHg).
FINTEPLA REMS Program (see Boxed Warning): FINTEPLA is available only through a restricted distribution program called the FINTEPLA Risk Evaluation and Mitigation Strategy (REMS) Program. Prescribers must be certified by enrolling in the FINTEPLA REMS. Prescribers must counsel patients receiving FINTEPLA about the risk of VHD and PAH, how to recognize signs and symptoms of VHD and PAH, the need for baseline (pretreatment) and periodic cardiac monitoring via echocardiogram during FINTEPLA treatment, and cardiac monitoring after FINTEPLA treatment. Patients must enroll in the FINTEPLA REMS and comply with ongoing monitoring requirements. The pharmacy must be certified by enrolling in the FINTEPLA REMS and must only dispense to patients who are authorized to receive FINTEPLA. Wholesalers and distributors must only distribute to certified pharmacies. Further information is available at www.FinteplaREMS.com or by telephone at 1-877-964-3649.
Decreased Appetite and Decreased Weight: FINTEPLA can cause decreases in appetite and weight. Decreases in weight appear to be dose related. Approximately half of the patients with LGS and most patients with DS resumed the expected measured increases in weight during the open-label extension studies. Weight should be monitored regularly during treatment with FINTEPLA, and dose modifications should be considered if a decrease in weight is observed.
Somnolence, Sedation, and Lethargy: FINTEPLA can cause somnolence, sedation, and lethargy. Other central nervous system (CNS) depressants, including alcohol, could potentiate these effects of FINTEPLA. Prescribers should monitor patients for somnolence and sedation and should advise patients not to drive or operate machinery until they have gained sufficient experience on FINTEPLA to gauge whether it adversely affects their ability to drive or operate machinery.
Suicidal Behavior and Ideation: Antiepileptic drugs (AEDs), including FINTEPLA, increase the risk of suicidal thoughts or behaviors in patients taking these drugs for any indication. Patients treated with an AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behaviors, or any unusual changes in mood or behavior.
Anyone considering prescribing FINTEPLA or any other AED must balance the risk of suicidal thoughts or behaviors with the risks of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behaviors. Should suicidal thoughts and behaviors emerge during treatment, consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.
Withdrawal of Antiepileptic Drugs: As with most AEDs, FINTEPLA should generally be withdrawn gradually because of the risk of increased seizure frequency and status epilepticus. If withdrawal is needed because of a serious adverse reaction, rapid discontinuation can be considered.
Serotonin Syndrome: Serotonin syndrome, a potentially life-threatening condition, may occur with FINTEPLA, particularly during concomitant administration of FINTEPLA with other serotonergic drugs, including, but not limited to, selective serotonin-norepinephrine reuptake inhibitors (SNRIs), selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants (TCAs), bupropion, triptans, dietary supplements (eg, St. John’s Wort, tryptophan), drugs that impair metabolism of serotonin (including monoamine oxidase inhibitors [MAOIs], which are contraindicated with FINTEPLA), dextromethorphan, lithium, tramadol, and antipsychotics with serotonergic agonist activity. Patients should be monitored for the emergence of signs and symptoms of serotonin syndrome, which include mental status changes (eg, agitation, hallucinations, coma), autonomic instability (eg, tachycardia, labile blood pressure, hyperthermia), neuromuscular signs (eg, hyperreflexia, incoordination), and/or gastrointestinal symptoms (eg, nausea, vomiting, diarrhea). If serotonin syndrome is suspected, treatment with FINTEPLA should be stopped immediately and symptomatic treatment should be started.
Increase in Blood Pressure: FINTEPLA can cause an increase in blood pressure. Rare cases of significant elevation in blood pressure, including hypertensive crisis, has been reported in adult patients treated with fenfluramine, including patients without a history of hypertension. In clinical trials for DS and LGS of up to 3 years in duration, no pediatric or adult patient receiving FINTEPLA developed hypertensive crisis. Monitor blood pressure in patients treated with FINTEPLA.
Glaucoma: Fenfluramine can cause mydriasis and can precipitate angle closure glaucoma. Consider discontinuing treatment with FINTEPLA in patients with acute decreases in visual acuity or ocular pain.
ADVERSE REACTIONS
The most common adverse reactions observed in DS studies (incidence at least 10% and greater than placebo) were decreased appetite; somnolence, sedation, lethargy; diarrhea; constipation; abnormal echocardiogram; fatigue, malaise, asthenia; ataxia, balance disorder, gait disturbance; blood pressure increased; drooling, salivary hypersecretion; pyrexia; upper respiratory tract infection; vomiting; decreased weight; fall; status epilepticus.
The most common adverse reactions observed in the LGS study (incidence at least 10% and greater than placebo) were diarrhea; decreased appetite; fatigue; somnolence; vomiting.
DRUG INTERACTIONS
Strong CYP1A2, CYP2B6, or CYP3A Inducers: Coadministration with strong CYP1A2, CYP2B6, or CYP3A inducers will decrease fenfluramine plasma concentrations. If coadministration of a strong CYP1A2, CYP2B6, or CYP3A inducer with FINTEPLA is necessary, monitor the patient for reduced efficacy and consider increasing the dosage of FINTEPLA as needed. If a strong CYP1A2, CYP2B6, or CYP3A inducer is discontinued during maintenance treatment with FINTEPLA, consider gradual reduction in the FINTEPLA dosage to the dose administered prior to initiating the inducer.
Strong CYP1A2 or CYP2D6 Inhibitors: Coadministration with strong CYP1A2 or CYP2D6 inhibitors will increase fenfluramine plasma concentrations. If FINTEPLA is coadministered with strong CYP1A2 or CYP2D6 inhibitors, the maximum daily dosage of FINTEPLA is 20 mg. If a strong CYP1A2 or CYP2D6 inhibitor is discontinued during maintenance treatment with FINTEPLA, consider gradual increase in the FINTEPLA dosage to the dose recommended without CYP1A2 or CYP2D6 inhibitors. If FINTEPLA is coadministered with stiripentol and a strong CYP1A2 or CYP2D6 inhibitor, the maximum daily dosage of FINTEPLA is 17 mg.
USE IN SPECIFIC POPULATIONS
In patients with severe impairment of kidney function (estimated glomerular filtration rate [eGFR]) 15 to 29 mL/min/1.73m2, dosage adjustments are recommended. FINTEPLA has not been studied in patients with kidney failure (eGFR <15 mL/min/1.73m2).
Combined molar exposures of fenfluramine and norfenfluramine were increased in subjects with various degrees of hepatic impairment (Child-Pugh Class A, B, and C), necessitating a dosage adjustment in these patients.
To report SUSPECTED ADVERSE REACTIONS, contact UCB, Inc. at 1 844-599-2273 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information, including Boxed Warning, for additional Important Safety Information.
Important Safety Information about BRIVIACT® (brivaracetam) CV in the US
INDICATION
BRIVIACT® (brivaracetam) CV is indicated for the treatment of partial-onset seizures in patients 1 month of age and older.11
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Suicidal Behavior and Ideation: Antiepileptic drugs, including BRIVIACT, increase the risk of suicidal behavior and ideation. Monitor patients taking BRIVIACT for the emergence or worsening of depression; unusual changes in mood or behavior; or suicidal thoughts, behavior, or self-harm. Advise patients, their caregivers, and/or families to be alert for these behavioral changes and report them immediately to a healthcare provider.
Neurological Adverse Reactions: BRIVIACT causes somnolence, fatigue, dizziness, and disturbance in coordination. Monitor patients for these signs and symptoms and advise them not to drive or operate machinery until they have gained sufficient experience on BRIVIACT.
Psychiatric Adverse Reactions: BRIVIACT causes psychiatric adverse reactions, including non-psychotic and psychotic symptoms in adult and pediatric patients. Advise patients to report these symptoms immediately to a healthcare provider.
Hypersensitivity: BRIVIACT can cause hypersensitivity reactions. Bronchospasm and angioedema have been reported. Discontinue BRIVIACT if a patient develops a hypersensitivity reaction after treatment. BRIVIACT is contraindicated in patients with a prior hypersensitivity reaction to brivaracetam or any of the inactive ingredients.
Withdrawal of Antiepileptic Drugs: As with all antiepileptic drugs, BRIVIACT should generally be withdrawn gradually because of the risk of increased seizure frequency and status epilepticus.
DOSING CONSIDERATIONS
Dose adjustments are recommended for patients with all stages of hepatic impairment.
When BRIVIACT is co-administered with rifampin, an increase in the BRIVIACT dose is recommended.
ADVERSE REACTIONS
In adult adjunctive therapy placebo-controlled clinical trials, the most common adverse reactions (at least 5% for BRIVIACT and at least 2% more frequently than placebo) were somnolence and sedation, dizziness, fatigue, and nausea and vomiting symptoms. Adverse reactions reported in clinical studies of pediatric patients were generally similar to those in adult patients. Adverse reactions with BRIVIACT injection in adult and pediatric patients were generally similar to those observed with BRIVIACT tablets. Other adverse events that occurred in adult patients who received BRIVIACT injection included dysgeusia, euphoric mood, feeling drunk, and infusion site pain.
BRIVIACT is a Schedule V controlled substance.
Please refer to the full Prescribing Information.
Important Safety Information about VIMPAT® (lacosamide) CV in the US5
INDICATION
VIMPAT® is indicated for the treatment of partial-onset seizures in patients 1 month of age and older. VIMPAT is indicated as adjunctive therapy in the treatment of primary generalized tonic-clonic seizures in patients 4 years of age and older.13
VIMPAT IMPORTANT SAFETY INFORMATION
VIMPAT is associated with important warnings and precautions including suicidal behavior and ideation, dizziness and ataxia, cardiac rhythm and conduction abnormalities, syncope, and Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), also known as multi-organ hypersensitivity.
Partial-Onset Seizures
In the adult adjunctive placebo-controlled trials for partial-onset seizures, the most common adverse reactions (≥10% and greater than placebo) were dizziness, headache, nausea, and diplopia. In the adult monotherapy clinical trial, adverse reactions were generally similar to those observed and attributed to drug in adjunctive placebo-controlled trials, with the exception of insomnia (observed at a higher rate of ≥2%). Pediatric adverse reactions were similar to those seen in adult patients.
Primary Generalized Tonic-Clonic Seizures
In the adjunctive therapy placebo-controlled trial for primary generalized tonic-clonic seizures, the adverse reactions were generally similar to those that occurred in the partial-onset seizures trials. The adverse reactions most commonly reported were dizziness, somnolence, headache, and nausea.
The adverse reactions associated with VIMPAT injection in adult patients with primary generalized tonic-clonic seizures are expected to be similar to those seen in adults with partial-onset seizures. The adverse reactions associated with VIMPAT injection in pediatric patients are expected to be similar to those noted in adults. Infusion times less than 30 minutes were not adequately studied in pediatric patients.
VIMPAT contains lacosamide, a Schedule V controlled substance.
Please refer to the full Prescribing Information.
BRIVIACT®, FINTEPLA®, RYSTIGGO®, and ZILBRYSQ® are registered trademarks of the UCB Group of Companies. VIMPAT® is a registered trademark used under license from Harris FRC Corporation. Staccato® is a registered trademark of Alexza Pharmaceuticals, Inc., and is used by UCB Pharma under license.
About UCB
UCB, Brussels, Belgium (www.ucb.com) is a global biopharmaceutical company focused on the discovery and development of innovative medicines and solutions to transform the lives of people living with severe diseases of the immune system or of the central nervous system. With approximately 8,600 people in approximately 40 countries, the company generated revenue of €5.5 billion in 2022. UCB is listed on Euronext Brussels (symbol: UCB). Follow us on Twitter: @UCB_news.
Forward looking statements
This press release may contain forward-looking statements including, without limitation, statements containing the words “believes”, “anticipates”, “expects”, “intends”, “plans”, “seeks”, “estimates”, “may”, “will”, “continue” and similar expressions. These forward-looking statements are based on current plans, estimates and beliefs of management. All statements, other than statements of historical facts, are statements that could be deemed forward-looking statements, including estimates of revenues, operating margins, capital expenditures, cash, other financial information, expected legal, arbitration, political, regulatory or clinical results or practices and other such estimates and results. By their nature, such forward-looking statements are not guarantees of future performance and are subject to known and unknown risks, uncertainties and assumptions which might cause the actual results, financial condition, performance or achievements of UCB, or industry results, to differ materially from those that may be expressed or implied by such forward-looking statements contained in this press release. Important factors that could result in such differences include: the global spread and impact of COVID-19, changes in general economic, business and competitive conditions, the inability to obtain necessary regulatory approvals or to obtain them on acceptable terms or within expected timing, costs associated with research and development, changes in the prospects for products in the pipeline or under development by UCB, effects of future judicial decisions or governmental investigations, safety, quality, data integrity or manufacturing issues; potential or actual data security and data privacy breaches, or disruptions of our information technology systems, product liability claims, challenges to patent protection for products or product candidates, competition from other products including biosimilars, changes in laws or regulations, exchange rate fluctuations, changes or uncertainties in tax laws or the administration of such laws, and hiring and retention of its employees. There is no guarantee that new product candidates will be discovered or identified in the pipeline, will progress to product approval or that new indications for existing products will be developed and approved. Movement from concept to commercial product is uncertain; preclinical results do not guarantee safety and efficacy of product candidates in humans. So far, the complexity of the human body cannot be reproduced in computer models, cell culture systems or animal models. The length of the timing to complete clinical trials and to get regulatory approval for product marketing has varied in the past and UCB expects similar unpredictability going forward. Products or potential products, which are the subject of partnerships, joint ventures or licensing collaborations may be subject to differences disputes between the partners or may prove to be not as safe, effective or commercially successful as UCB may have believed at the start of such partnership. UCB’s efforts to acquire other products or companies and to integrate the operations of such acquired companies may not be as successful as UCB may have believed at the moment of acquisition. Also, UCB or others could discover safety, side effects or manufacturing problems with its products and/or devices after they are marketed. The discovery of significant problems with a product similar to one of UCB’s products that implicate an entire class of products may have a material adverse effect on sales of the entire class of affected products. Moreover, sales may be impacted by international and domestic trends toward managed care and health care cost containment, including pricing pressure, political and public scrutiny, customer and prescriber patterns or practices, and the reimbursement policies imposed by third-party payers as well as legislation affecting biopharmaceutical pricing and reimbursement activities and outcomes. Finally, a breakdown, cyberattack or information security breach could compromise the confidentiality, integrity and availability of UCB’s data and systems.
Given these uncertainties, you should not place undue reliance on any of such forward-looking statements. There can be no guarantee that the investigational or approved products described in this press release will be submitted or approved for sale or for any additional indications or labelling in any market, or at any particular time, nor can there be any guarantee that such products will be or will continue to be commercially successful in the future.
UCB is providing this information, including forward-looking statements, only as of the date of this press release and it does not reflect any potential impact from the evolving COVID-19 pandemic, unless indicated otherwise. UCB is following the worldwide developments diligently to assess the financial significance of this pandemic to UCB. UCB expressly disclaims any duty to update any information contained in this press release, either to confirm the actual results or to report or reflect any change in its forward-looking statements with regard thereto or any change in events, conditions or circumstances on which any such statement is based, unless such statement is required pursuant to applicable laws and regulations.
Additionally, information contained in this document shall not constitute an offer to sell or the solicitation of an offer to buy any securities, nor shall there be any offer, solicitation or sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to the registration or qualification under the securities laws of such jurisdiction.
US-BR-2400072
References:
- RYSTIGGO® US PI. https://www.ucb.com/sites/default/files/2023-08/Rystiggo_Prescribing_Information_USA.pdf. (Accessed: April 2024).
- ZILBRYSQ® US PI. https://www.ucb.com/sites/default/files/2024-01/Zilbrysq_PI_27oct2023.pdf. (Accessed: April 2024).
- FINTEPLA ® US PI. https://www.ucb.com/sites/default/files/2023-12/Fintepla_Current_COL_12_2023.pdf. (Accessed: April 2024).
- BRIVIACT ® US PI. https://www.ucb-usa.com/briviact-prescribing-information.pdf. (Accessed: April 2024).
- VIMPAT ® US PI. https://www.ucb-usa.com/vimpat-prescribing-information.pdf. (Accessed: April 2024).
Choose Country
- Global Site – English
- Australia – English
- België – Engels
- Belgique – Anglais
- Brasil – Português
- България – Български
- Canada – English
- Canada – Français
- 中国 – 中文
- Česká Republika – Angličtina
- Danmark – Engelsk
- Deutschland – Deutsch
- France – Français
- España – Español
- Ελλάδα – Ελληνικά
- India – English
- Ireland – English
- Italia – Inglese
- 日本 – 日本語
- Казахстан – ағылшын тілі
- 한국 – 한국어
- Luxembourg – Anglais
- Luxemburg – Engels
- Magyarország – Angol
- México & Latinoamérica – Español
- Nederland – Engels
- New Zeeland – English
- Norge – Engelsk
- Österreich – Deutsch
- Polska – Polski
- Portugal – Inglês
- România – Engleză
- Россия – Русский
- Slovensko – Anglický
- Suomi – Englanti
- Sverige – Engelska
- Schweiz – Deutsch
- Suisse – Français
- Türkiye – Türkçe
- Україна – Англійська
- United Kingdom – English
- U.S.A. – English